Before you start a medical wearable device project, consider the following challenges and suggestions on how to address them.
Diana Eitzman, Ph.D. and Kris Godbey
Skin is unlike any other substrate. It sweats, grows hair, secretes oil, harbors bacteria, constantly sheds old cells, regenerates new ones and changes with health, environment and age – characteristics that are far from universal.
Understanding these unique skin factors and the design challenges they present prior to delving into a stick-to-skin device project will help steer the product development process down a clearer path, the benefits of which will be felt by manufacturers, engineers and end users alike. Not only will implementation of this knowledge lower the likelihood of irritating or damaging skin – it will also work towards a more cost-effective and time-efficient process. Not addressing these issues from the get-go can elongate a project’s timeline or cause the budget to prematurely run dry.
The good news is that these negative outcomes are preventable.
- Understanding the science of skin
We recognize that an engineer’s first consideration may not be how well and how long an adhesive will stick on an elderly person’s skin versus a two-month-old baby, but it’s critical to understand the numerous skin variables of the end user. Differences in skin elasticity, moisture, oil secretion, and other characteristics can all impact what type of adhesive will perform the best for a device design. Healthy, young skin is less vulnerable, but the reality is that not all skin can handle the same level of sticking power.

How well and how long an adhesive will stick on an elderly person’s skin versus a two-month-old baby is one of the critical factors to the design process and selecting the proper adhesive.
Beyond the human factors listed above, there are other factors such as culture, diet, environment, age, and overall health of the end user to consider. The location of the device on the body also plays into this. The skin on your chest is different than the skin on your foot, so the adhesive’s needs and properties will have to reflect those differences.
- Communicating effectively with the customer
As obvious as it might sound, it’s incredibly important for the design team and their customer to be on the same page, from the beginning, on adhesive expectations. We’ve heard about too many projects that stumble out of the gate because the perceived definition of, say, durability meant something different to the engineer than it did for the customer. Miscommunications, such as these, oftentimes are linked back to the two parties speaking different languages. Engineering jargon must be translated to an accessible language comprehended by everyone.
We recommend explaining requirements as clearly as possible and keep open lines of communication. Of course clearly defined specifications can also help overcome this obstacle, and reduce the product development cycle.
- Finding the right adhesive for each application
Adhesive and substrate choice affect a smooth design and development process, as not all adhesives were meant to pair with every substrate. Finding the ideal adhesive-substrate combination on a project-by-project basis can help prevent the need to repeat clinical trials or studies, and such end-use consequences as irritated skin, premature device fall-off or difficult removal.
Below is a brief rundown of commonly used substrates in medical device applications.
Polyester: Easy to adhere to and, although rather inflexible, can be molded into a part, fiber or film. It is also clear, hard and protective.
Polyethylene: Soft to the touch, is relatively easy to work with and conformable. Polyethylenes are also reasonably priced.
Polyurethane: Ideal for wound dressings and other medical needs because they’re breathable, soft, and offer great flexibility.
PVC: While medical tubing is still mostly made out of it, PVC has its own set of adhesion difficulties. It’s hard to dispose of and can cause some tapes to become soft and soupy because of plasticizer migration. But it is flexible and clear.
Silicone: A popular choice for medical devices, but can be difficult to stick to. There are alternative materials that open up adhesive choices and exhibit similar characteristics known and loved by developers.
What’s exciting about the growing interest in designing wearable medical devices, is that new applications are continuously being developed and tested to optimize performance. The industry is making strides in strong adhesion and durability without sacrificing breathability and comfort expectations.
Extending wear times
The skin’s first layer, the stratum corneum, is the one that adhesives stick to and the one that completely replaces itself about every two weeks. With a constantly shifting substrate, extended wear time can be tricky to achieve.
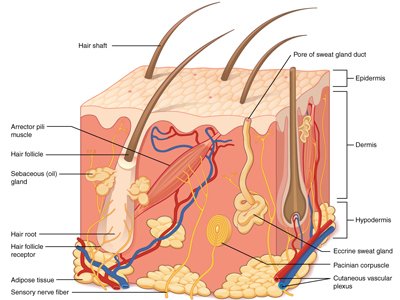
Differences in skin elasticity, moisture, oil secretion, and other characteristics impact what type of adhesive will perform the best for a device design. The skin’s first layer, the stratum corneum, is the one that adhesives stick to and the one that completely replaces itself about every two weeks. With a constantly shifting substrate, extended wear time can be tricky to achieve.
Tricky, but not impossible.
To give engineers insight into wear time with current adhesive options, 3M recently conducted 8-, 15- and 21-day adhesive wear time trials on human volunteers. These trials led to best practice recommendations in choosing the proper adhesive for a medical device project. Based on these, similar studies, and 3M’s more than five decades of experience in medical device adhesives, 3M engineers compiled some of the most critical questions to ask when determining wear time. These include:
How long does the device need to stay on?
Who is the intended end user (i.e. newborn, child, adult, senior)?
Where on the body will the device be attached?
It’s important to not over design. Only ask an adhesive to do what the device requires, not more, especially when it comes to wear time. If what’s being secured is only required to stay on for a day or two, designers shouldn’t pick an adhesive that’s meant to stay on for a week. This can heighten the risk of a Medical Adhesive-Related Skin Injury, or MARSI, when the adhesive-to-skin bond is stronger than the skin-to-skin attachment.
The effects of sterilization
While sterilization plays a critical role in killing bacteria and microbes, the process can produce negative outcomes, such as affecting adhesive performance and device function, if not properly addressed.
Two common radiation-based sterilization methods are e-beam and gamma. They’re both effective in their intended purpose, but can cause some plastics to turn yellow, acrylate adhesives to become firmer and synthetic rubber-based adhesives to get stickier, among other adhesive property changes. Sterilization processes have also been known to make liners more difficult to release from the adhesive, or cause a worst-case scenario – liners that don’t remove at all.
Understanding and evaluating sterilization effects will not only avoid these issues, but also help keep cost and time overruns in check during the production cycle.
Pairing adhesive with backing
As stated before, skin is dynamic. It’s a living organ. Just like any other living thing, it can start to feel trapped if an obstructive foreign device is suffocating it, and it’ll react accordingly. With medical devices, that reaction is typically irritation and increased moisture in the covered area forcing the foreign device to no longer adhere to the skin, but it can also lead to further skin damage. Finding a breathable backing and adhesive solution optimized for the application will help keep the skin from having a negative reaction.
Backing and adhesive pairings can impact devices in numerous other ways, as well. For long-term wear, moisture management while maintaining breathability is desirable, as well as flexible backings to allow skin to perform as it normally does. Some extended wear devices may benefit if the skin contact tape extends beyond the device being secured, which will also allow the skin to move and flex around it.
Engineers must collaborate with their customers to work through wearable projects’ complexities – including the important features of medical adhesives – and determine how they apply to their project. It’s also important to note that engineers don’t have to navigate the terrain alone. Some adhesives experts offer support throughout the entire design and development process and are able to provide critical analysis of a project’s needs, discuss adhesive options, deliver design insights or help to translate jargon.
Addressing these challenges can be difficult, but the extra foresight will be worth it when the device succeeds in its intended function – improving the wearer’s quality of life.
3M
www.3m.com
Filed Under: Adhesives • epoxies, Medical-device manufacture




Tell Us What You Think!