
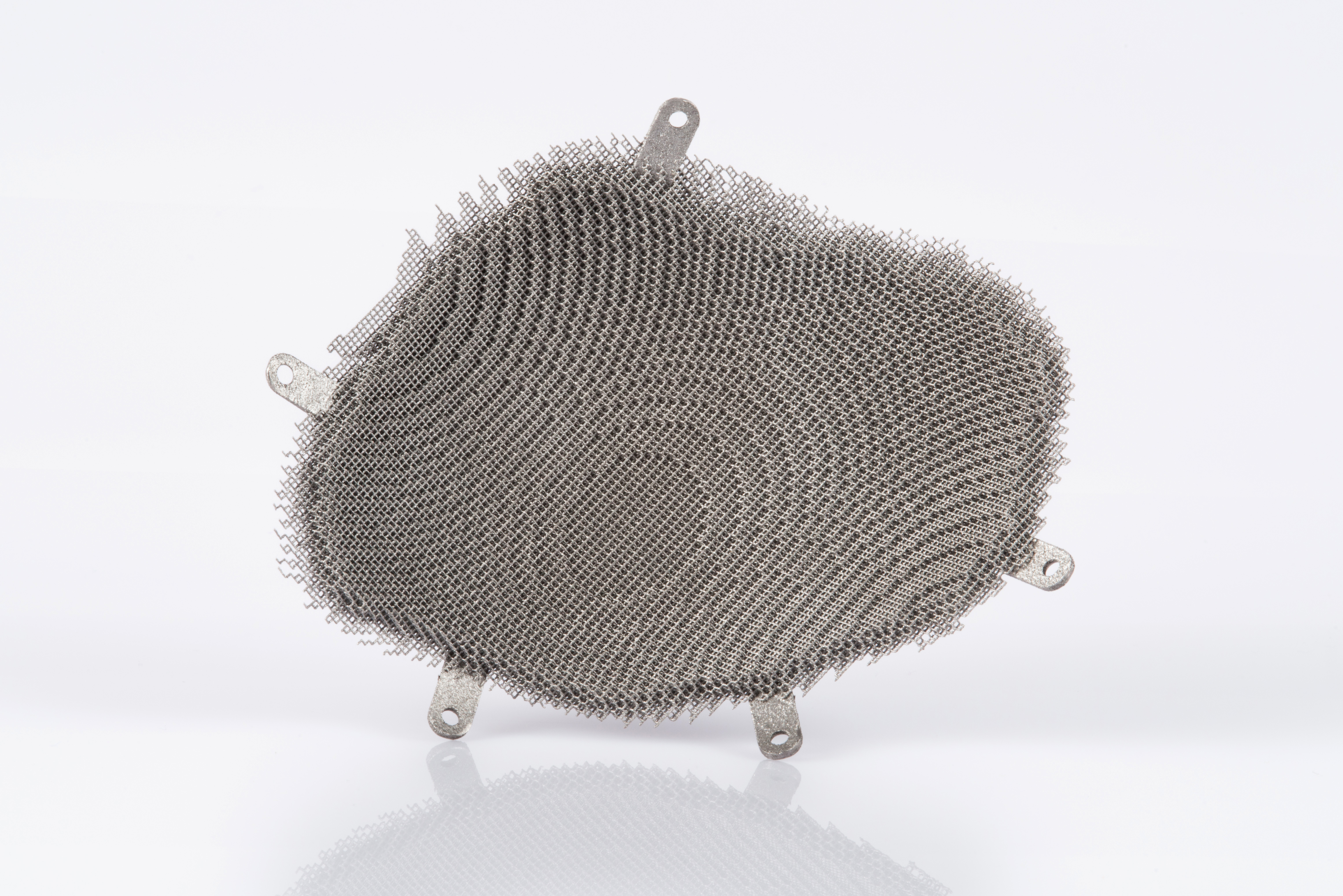
The porosity of the implant is 95 percent, so liquids can flow through with the least possible resistance and the bone tissue optimally coalesces with the outer edges (courtesy of EOS).
Cranial implants need to meet many highly stringent criteria. In the case of an Argentine patient who required a particularly large implant after stroke-related surgery, the project called for a level of design that was as close to perfect as one can imagine: from the highest degree of precision and compatibility to the integration of biological functions.
The 3D-printing service provider Alphaform AG relied on EOS technology to create this successful cranial implant, developed by partner firm Novax DMA.
Challenge
If a person requires an implant in the skull, then external factors—in particular, the implant itself—should aid, not hinder, the healing process. Most importantly, the implant should have the most perfect fit possible—a classic requirement of additive manufacturing (AM) applications.
The production process, which uses a laser to build up the material (in this case titanium) piece by piece, offers maximum individualization in both form and size.
The doctors involved provided medical technology experts from Novax DMA and Alphaform with further challenges to meet along the way.
High on the list, due to the size of the hole in the patient’s bone structure, was the integration of biological functions and the lowest possible degree of heat dissipation into the cerebral tissue.
Titanium has excellent biocompatibility with the human body. Nonetheless, because it is a metal, it could generate too much heat inside the body in the event of high exposure to sunlight. Another potential problem is that a titanium structure could be impermeable to tissue fluid from the brain.
In their requirement specifications, the doctors also stipulated special post-manufacturing processes for the implant. Controlled and optimal post-processing, particularly in terms of cleaning, facilitates the use of the part.
This is vital because stray particles might lead to possible infections or even rejection. In addition, absolute sterility is central to successful acceptance of the implant by the body.
The additive manufactured implant made of a biocompatible titanium alloy was placed in the skull of a patient in Argentina who required it after stroke-related surgery (courtesy of Novax DMA).
Solution
Only a porous structure would be capable of meeting the required characteristics. A lattice-structured implant with skull-integrated, screw-in fixings facilitates both the passage of fluids and fusion with the bone of the skull itself. In addition, such a design would have an insulation effect such that the heat dissipation into the cranial cavity would be minimized.
The dimensions of the pores themselves are approximately 1mm in size, while the cell-links are approx. 0.2mm thick.
Daniel Fiz, CEO of Novax DMA, says, “Time played an important role, since patients should receive their implants as quickly as possible. Once we had the dimensions data, we began construction immediately.” For the 3D design of the implant they employed software from Within Ltd. “It allowed us not only to define the basic form quickly, but also the porous structure itself,” explains Kaveh Mahdavi, business development at Within. As soon as the CAD work was complete, Alphaform took on the manufacture of the implant, using an EOSINT M 280 system from EOS. The build time was just a matter of hours.
“We had already successfully completed many products with the EOS system,” says Christoph Erhardt, director of additive manufacturing at Alphaform AG. “However, we are particularly proud of this implant, not only because of the precise realization of the form, but especially because we were able to optimize the cleaning processes.” Porous structures in particular, with their small interior hollow spaces, are extremely hard to clean. The process is relatively sensitive. Basically, Alphaform applied a multi-step process of abrasive and mechanical cleaning, rinsing, and ultrasound in order to meet the medically required standards.
Results
The perfectly tailored implant meets all clinical requirements. Its porosity level reaches 95 percent, which means liquids could flow through with the least possible resistance.
In addition, the bone tissue is able to penetrate the outer edges of the implant and grow together with it. At the same time, the metal is stable enough to return the patient to the desired level of normality in everyday life.
The structure, constructed in the form of a regular lattice, also provides the desired level of thermal conductivity—so the patient can even enjoy time in the sun.
Time to market is a key strength of AM in industry, but time is even more critical in medicine. The implant was in the operating theatre in just three weeks. The largest block of time was taken up by transportation, which took a week.
Data preparation and then construction were completed within just two and a half days. The remaining time was split among various processes, including logistics and coordination.
The two companies verified the purity levels of the implant through comprehensive measurements. Erhardt and his team carried out extensive tests, including particle and cytotoxicity testing. They also undertook a gas-chromatography analysis.
“All of the analyses confirmed that the implant produced through additive manufacturing fulfilled the necessary requirements to stabilize and protect the patient’s skull,” he says. “The one and a half hour surgery went smoothly. The patient left the hospital after two days and the wound was healed within three weeks. Since then, there have been no complications.”
Filed Under: Industrial automation




