By Boaz Eidelberg, Ph.D., Jim Monnich, Parker Daedal Engineered Solutions, Electromechanical Automation North America
Pathological scanners have unique requirements of fitting into a small space with custom restrictions. Standard stages cannot fulfill the needs of modern pathological scanners. But a new infrastructure for effective pathological scanner development does fit custom needs.
Scanner products typically require unique positioning specifications, which are not available with off-the-shelf products. The requirements often include tight geometrical constraints, high accuracy, high smoothness of motion, and long life. In addition they require high product consistency for high-volume manufacturing at a global competitive cost.

An example of a pathological scanner. The flatness of Parker scanners is on the order of submicron levels. This flatness performance is achieved by using linear bearing technologies that are specifically selected for this type of application.
Life science automation markets
End users of life science automated tools mainly include research labs, pharmaceutical companies and point of care (POC) sites such as hospitals and doctor offices. We define automation tools as life science process tools, which are designed to operate either as manual feed for machines in small labs and offices, or as fully automated systems with auto loaders that operate 24/7.
Point-of-care diagnostic tools provide health care consumers with faster, more comprehensive diagnostic services for preventive, curative or rehabilitation health care at a lower cost. These desktop tools are located at doctors’ offices, clinics and hospitals. They automatically take pre-prepared samples from the patient, load them into the tool and make them interact with biochips, which contain hundreds of thousands of micro wells with genomic and other reagents. Reaction is then sensed by various optical means and conclusions are made within minutes, rather than hours or days. Although in its infancy, this market is estimated to grow at 15% a year and is expected to be a major cornerstone of the heath care industry.
Automation technology, which supports R&D, pharmaceutical and POC users, include, among others, scanning microscopy, lab automation and microchip analyzers, respectively.
Microscopy: Much of life science research is done under the microscope, usually to analyze complex biological structures. These structures include, proteins, cells, genes, and tissues. Analysis is done on the complex interaction of these structures with submicron pathogens (such as bacteria and viruses) under the influence of curative and preventive drug molecules.
Automation may be done with scanning microscopes, which operate as part of a larger automated cell that in turn may include other equipment, such as load-unload robots and image processing controllers.
Lab Automation: Lab automation includes an array of tools that help the pharmaceutical industry expedite the process of drug discovery. According to the trade group Pharmaceutical Research and Manufacturing of America, it takes an estimated cost of $500 to $800 M and 10 to 15 years per successful drug discovery. The hit rate, from a molecule that enters the R&D phase to a drug approval for consumption by people, is 1 in 5,000 – 10,000, according to the same article. Automation therefore, has high value in optimizing the drug discovery process.
High throughput screening (HTS) is an example of a common automated process used in drug discovery. In this process, automated robots handle fluids and powders. They create and sense reactions between target biological molecules, such as sick cells with reagents and millions of molecules, which are drug candidates. Lab automation is typically done in automated cells, which include many ancillary tools such as Cartesian robots, articulating robots, XYZ stages, gantry stages, linear and rotary actuators, indexing tables, conveyors, storage cabinets, bar code readers, hot plates, mixers and shakers. The process is done in an orderly sequence, 24/7, using PLC and motion controllers.
Pathological Scanners: Pathological scanners are a subset of scanning microscopy. They provide similar information as scanning microscopes at a resolution on the order of hundreds of nanometers. Pathology scanners are used extensively in the study and monitoring of diseases. The scanning process includes a biological sample such as a tissue, which is placed on a glass substrate called a “slide.” Several slides are loaded into a cassette. The cassette is placed on an XY stage, which is mounted to the scanner frame. A vertical Z stage is mounted to the scanner frame above the XY stage, carrying the focusing optics. During the scanning process, the XY stages move the slides under the camera at a typical rate of 5 mm/s. The camera takes digital images within its field of view, which is on the order of 1 mm2. While the XY stages are moving, to cover the entire slide area, which is typically on the order of 50 mm x 25 mm, the Z axis focuses the camera and holds a stable position for a fraction of a second, while snap shots are being taken by the camera.
Scanner positioning stages must meet certain space restrictions. These restrictions are the result of market needs for a minimal footprint, given camera shape and size, illumination requirements, and special slide holder restrictions. These restrictions limit the choice of XYZ configurations and often require low profile, built in apertures and custom axes with miniature components. The key to success in the system-configuration phase is a close interaction between customer engineers and the stage manufacturer engineers. CAD files are often exchanged in the preliminary design phase to assure the design meets travel requirements of all axes, cable management, operation safety and accessibility for efficient assembly and field maintenance.
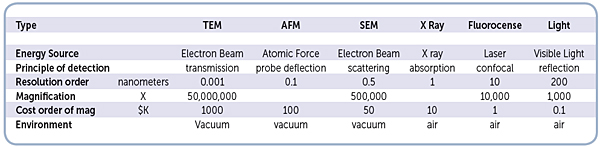
This chart consolidates various microscope technologies, which are commonly used in biotechnology related research.
Scanner XY stages
In scanning applications, the most important specifications of the XY stages are as follows:
- Flatness – Scanner flatness must be minimal to reduce the dynamic load from the Z axis auto focus
- Straightness – Scanner straightness is required to minimize overlap between scanning passes and increase process throughput
- Smoothness of motion – Smooth motion is required to assure the highest possible image resolution.
Flatness of motion is determined by several factors, including flatness of the slide and base surfaces of the stage and flatness of the linear rails. A typical flatness requirement for pathological scanners is on the order of sub micron per mm. For example, consider an X stage that scans at 5 mm/sec and has a flatness of ± 1 μm/mm. This flatness and scanning rate represent a position disturbance to the autofocus with an amplitude of 1 μm and a frequency of 5 Hz. This in turn implies that the required bandwidth of the Z stage should be at least 3 times higher than the disturbance frequency. It also implies that the natural frequency will be three times higher than the bandwidth.
Straightness is determined primarily by the rails and assembly. Parker straightness, for example, using high-precision rails and special assembly fixtures, is smaller than ±0.25 μm/mm.
Miniature linear motors drive the XY stages with linear encoders as feedback. Miniature linear motors have the advantage of being compact with low profile size and totally noncontact. They are therefore an advantage in applications of limited space, such as pathology scanners. Linear motors are virtually jitter- and friction-free actuators, resulting in high smoothness of motion and long life.
Scanner auto focus Z stage
The Z axis stage supports the optical head, including lenses and mounting bracket of the Z axis auto focus. The moving weight is on the order of 0.5 kg. The operation of the auto focus Z stage is key to the performance of the scanner and to the image clarity of the scanned objects. It has to have extremely high resolution, in the nanometer range, fast response, short settling time, stable holding position and repeatable operation. It also has to have a precision controller and very consistent performance from one stage to the other.
The following are alternative options for Z stage configuration:
- Stacked Piezo stage – These stages have very fast response and excellent position holding stability. Their limitations are in positioning hysteresis, which make the servo control nonlinear and more difficult to tune. They also have extremely short travel, on the order of a few hundred micrometers, which limits other tasks needed by the Z stage, such as mapping the entire XY range for Z height, or adopting to several chuck sizes or optics with different heights.
- Hybrid Piezo stages – These stages consist of a combination of a ball screw stage and a stacked piezo stage. The ball screw stage provides long travel at low accuracy. The precision-stacked piezo stage, which is mounted on top of the ball screw stage, provides holding stability and precise positioning. The advantage of this hybrid configuration is in increased travel with the benefits of the two positioning technologies used. Its disadvantage is its larger size, additional servo axis to control and difficult tuning of both stages.
- “Walking” Piezo stages – These stages have the advantage of miniature size, holding stability and long travel. Their disadvantage is in their change of performance over time. Most OEM users require that the system will be tuned with a consistent set of parameters for all similar stages. They expect all stages of the same type to act the same way over the lifetime of their systems.
- Voice coil or linear motor stages – These stages have the advantage of offering long travel, miniature size, frictionless operation, low maintenance and consistent performance over the lifetime of the stage. Their disadvantage is in the need for a counterbalance in vertical applications. Solutions exist to resolve this limitation, including, among others, a non-contact magnetic counterbalance with a uniform force constant throughout the travel.
- Encoder – To assure long-term performance uniformity, the Z stage encoder is made of Invar with a coefficient of thermal expansion of nearly zero. This characteristic, together with proprietary mounting procedures, assures long-term thermal stability despite possible variations in temperature during long-term scanning. Thermal variation may cause structural deformation, which elongates the encoder scale and introduces non-repeatable positioning errors.
- Bearing – The unique nature of repetitive micron distance moves, required by autofocus stages, causes specific complications for certain bearing types.
- Counterbalance – To uncouple the moving weight from the continuous force requirements of the Z motor, a counterbalance has to be used. Parker is using a non-contact magnetic counterbalance that has a uniform force constant, throughout the travel range of the stage, with repeatability of 0.1 µm.
Additional design considerations
It should be noted that for high smoothness of motion and short settling time, additional design considerations are recommended, as follows:
- Cable management should be designed to minimize rattling effects.
- Wires are shielded and grounded to common ground to reduce EMI and RFI noise.
- The equipment frame is isolated from ground disturbances.
- Structural damping is optimized to attenuate high-frequency noise amplification.
- The stage structure and machine frame natural frequency are designed to meet the required move and settle frequency response.
- The controller capability must be matched to the required frequency response.
- Proper servo filtering is used to attenuate high frequency noise.
In addition, note that to meet challenging design and performance objectives under tight constrains of budget and schedule, the following infrastructure building blocks are essential:
- A team of engineering problem solvers that can quickly evaluate alternative configurations and predict cost and performance tradeoffs of candidate solutions.
- Manufacturing tools and resources are needed to quickly conduct feasibility tests of the selected configuration. These tests are done to assure that there are no knowledge gaps between conceptual solutions and full-scale manufacturing. These tests are also intended to reduce development risks.
- Close interaction with the customer engineering team.
Manufacturability
Developing a cost-effective positing system is only the first step in a successful business development program. The next important consideration is to bridge the gap between a working prototype and a full-scale production environment. The recommended procedures are based on years of experience in developing and manufacturing thousands of positioning systems. They are as follows:
- Highly organized production files, including BOM, drawings, material sources, assembly and test instructions and a dedicate team for JIT manufacturing.
- For large OEM production, a dedicated manufacturing cell is developed which employs state-of-the-art, lean, manufacturing principals and quality plans throughout the manufacturing process. In many cases a final test stand, using the end customer structure, is used to test parameters and variables, which are critical to operation performance.
- Acceptance Test procedure (ATP) – The test procedure is agreed upon with the customer during the development phase. ATP is intended to assure, that all systems, which leave the factory, meet critical performance requirements. It is also intended to assure, that system parameters are consistent with parameters of other similar products for a long-term product consistency.
Parker Daedal Engineered Solutions
www.parkermotion.com/engineereddesigns
Filed Under: Stages • gantries, Medical-device manufacture, Encoders • linear, LINEAR MOTION, MECHANICAL POWER TRANSMISSION




Tell Us What You Think!