Population growth, an increasing rate of chronic disease and economic factors are behind the growing demand for medical electrical equipment for use in home settings. Concurrently, the level of knowledge or understanding by the patient or caregiver, environmental factors and unpredictable factors are placing greater demands on the safety, durability and operability of medical equipment used in home settings.

First introduced in 2010, IEC 60601-1-11 is the current version of collateral standard IEC 60601-1 that applies to the basic safety and essential performance of medical electrical equipment used in home settings. Effective June 30, 2013 in the European Union and December 31, 2013 in the U.S., the new standard differs from the previous edition(s) in several key ways. It now requires a ‘Class II’ electrical categorization of medical equipment and systems for home use and expands the definition of the ‘home environment’ to include European nursing homes. As a result of these changes, manufacturers will now need to re-evaluate the design of legacy products and in many cases, re-design those and future products to meet these new and evolving standards.
The FDA defines a home use medical device as “a device intended for users in a non-clinical or transitory environment, which is managed partly or wholly by the user, requires adequate labeling for the user, and may require training for the user by a healthcare professional in order to be used safely and effectively. This includes permanently and temporarily implanted devices and any type of equipment that a person may use to recover and rehabilitate.” Because healthcare recipients expect to be able to stay independent, mobile, and active, the term “home use” extends beyond the home, per se, to encompass all environments in which a person plans to use his or her medical device in day-to-day life.
Issues with home healthcare
Because the home healthcare environment is fundamentally different than the clinical environment, home use of medical devices presents a variety of unique challenges, many of which have the potential to impact patient safety. For instance, a home’s age, structure and location may not have the electrical outlets required for some medical devices. Large equipment may not fit in smaller, older homes with narrower doors, hallways, etc. Additionally, pets and children may directly interfere with device operation, by tugging on, removing or ripping cords; changing settings; and more. Sanitation issues need to be taken into consideration such as managing medical waste and plumbing (city vs. well water, for instance). Finally, other items such as temperature extremes, dust, ventilation, fire and tripping hazards, electromagnetic interference, lighting, and more need to be considered. A hospital or facility will have taken care of all of these things already but it is completely different in the home.
The standard: IEC 60601-1-11
While “IEC 60601-1-11: 2010” is the international standard detailing requirements for medical electrical equipment and medical electrical systems used in the home healthcare environment, deviations related to local codes are given according to regional levels. The chart on the following page titled “Regional Deviations to International Standard IEC 60601-1-11: 2010” spells them out further.
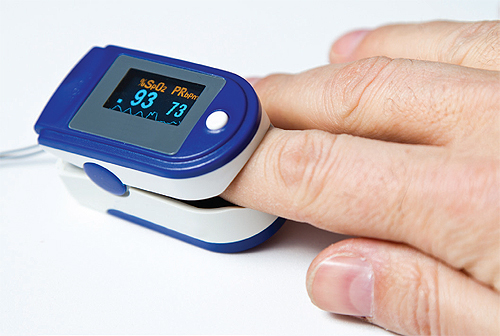
The growing use of home medical devices has led to changes in the IEC 60601-1-11 standard regulating their safety and performance.
Key changes to new standard
IEC 60601-1-11 differs from the previous edition(s) in the following key ways:
• Class I vs. Class II Designation — As studies estimate that 60% of the homes in Europe and 40% of those in the U.S. have no reliable earth ground wires, IEC 60601-1-11 requires that all medical devices for home use which are not being installed permanently in the home by a licensed electrician (a requirement of Class I products), now fall under the Class II designation, which does not rely upon earth ground for protection. With their two-prong, double insulated AC input, Class II classification for medical devices in the home setting is intended to protect non-professional users from electrical shock by approaching all home settings as if they do not have protective earth ground wiring. Within their Class II classification, applied parts shall be either Type ‘BF’ (floating applied parts) or Type ‘CF’ (a more stringent level of protection required for parts in cardiac equipment intended to come into contact with the heart).
• Definition of “Nursing Home” by Region – In the specific context of nursing homes, IEC 60601-1-11 now makes a distinction in the definition of product class requirements between the U.S. and Europe, e.g., while nursing homes in the U.S. are considered ‘professional’ environments where subsequent products must meet Class I requirements, nursing homes in Europe are now considered ‘home’ environments, which therefore requires that these products meet Class II requirements. As a result, U.S. medical device manufacturers selling into the European nursing home market will need to reconsider the way they think about and design products for that setting, as these devices are no longer destined for a professional setting and now need to meet the requirements of Class II home-use standards.

Because “home use” encompasses all environments in which a person plans to use a medical device in day-to-day life, these devices must meet certain electrical regulations.
• New Usability Engineering Process— From a product design perspective, IEC 60601-1-11 (Clause 9) introduced new concepts that design engineers must now consider as part of the Usability Engineering Process and File when manufacturing medical products for home use. Protection against all of the following hazards and possibilities must now be considered and designed into medical devices and equipment for home use:
• Changes of Controls
• Confusion in Operation Modes
• Unexpected Movement
• Transfer of Energy/Substances
• Potential of Disconnection
• Exposure to Biological Materials
• Improper/Unsafe Operation
• Parts Inhaled/Swallowed
• Avoiding Failure — In light of the fact that it can take as long as 12-18 months to secure approvals for medical electrical equipment and systems for home use, it is critical for manufacturers to comply with all requirements of IEC 60601-1-11 to ensure a smooth and efficient process to market. While the products themselves must be appropriately redesigned to meet new safety, performance and testing requirements, proper documentation is equally important; the majority of medical device products for home use today fail to secure approval because of incorrect or non-compliant documentation related to their manuals and markings, which can create as many delays and additional layers of review as product non-compliance itself.

Regional Deviations to International Standard IEC 60601-1-11: 2010
To read a more in-depth version of this article, including a look at more key changes to the standard, read the white paper at www.engineeringwhitepapers.com.
Intertek
www.intertek.com
Filed Under: Medical-device manufacture




Tell Us What You Think!