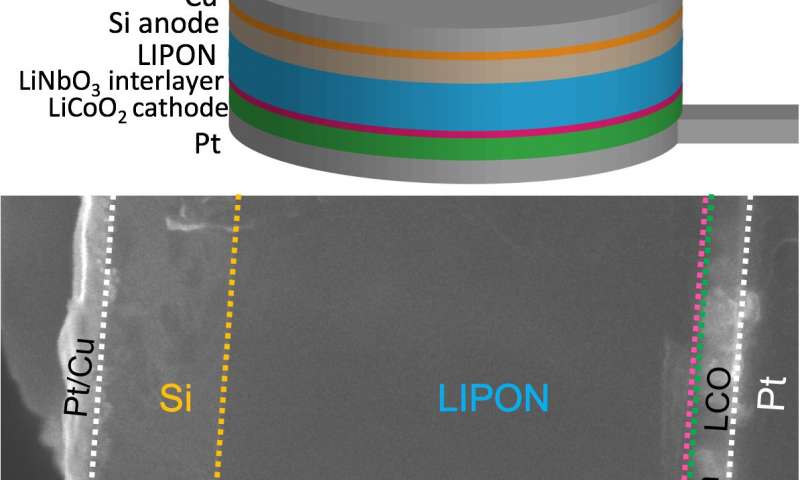
Schematic of full battery cell architecture and cross-sectional microscopic image of the actual battery
Research at Sandia National Laboratories has identified a major obstacle to advancing solid-state lithium-ion battery performance in small electronics: the flow of lithium ions across battery interfaces.
Sandia’s three-year Laboratory Directed Research and Development project investigated the nanoscale chemistry of solid-state batteries, focusing on the region where electrodes and electrolytes make contact. Most commercial lithium-ion batteries contain a liquid electrolyte and two solid electrodes, but solid-state batteries instead have a solid electrolyte layer, allowing them to last longer and operate more safely.
“The underlying goal of the work is to make solid-state batteries more efficient and to improve the interfaces between different materials,” Sandia physicist Farid El Gabaly said. “In this project, all of the materials are solid; we don’t have a liquid-solid interface like in traditional lithium-ion batteries.”
The research was published in a Nano Letters paper titled, “Non-Faradaic Li+ Migration and Chemical Coordination across Solid-State Battery Interfaces.” Authors include Sandia postdoctoral scientist Forrest Gittleson and El Gabaly. The work was funded by the Laboratory Directed Research and Development program, with supplemental funding by the Department of Energy’s Office of Science.
El Gabaly explained that in any lithium battery, the lithium must travel back and forth from one electrode to the other when it is charged and discharged. However, the mobility of lithium ions is not the same in all materials and interfaces between materials are a major obstacle.
Speeding up the intersection
El Gabaly compares the work to figuring out how to make traffic move quickly through a busy intersection.
“For us, we are trying to reduce the traffic jam at the junction between two materials,” he said.
El Gabaly likened the electrode-electrolyte interface to a tollbooth or merge on a freeway.
“We are essentially taking away the cash tolls and saying everybody needs to go through the fast track, so you’re smoothing out or eliminating the slowdowns,” he said. “When you improve the process at the interface you have the right infrastructure for vehicles to pass easily. You still have to pay, but it is faster and more controlled than people searching for coins in the glove box.”
There are two important interfaces in solid state batteries, he explained, at the cathode-electrolyte junction and electrolyte-anode junction. Either could be dictating the performance limits of a full battery.
Gittleson adds, “When we identify one of these bottlenecks, we ask, ‘Can we modify it?’ And then we try to change the interface and make the chemical processes more stable over time.”
Filed Under: Materials • advanced




