From design and manufacturing, all the way through to secondary operations and commercialization, Phillips-Medisize is among the more successful complete medical device manufacturers in the United States.
The range of in-house capabilities, services, and expertise appealed to a major medical original equipment manufacturer that chose to partner with Phillips-Medisize in the design of a new medical device for a minimally invasive surgical procedure.
The customer involved Phillips-Medisize engineering team at the onset. The task was to create a plastic handle that would operate in concert with two neighboring components, as well as a surgeon. The handle would have to reach – and ideally exceed – parity with competitors’ similar products, which were already available in the marketplace.
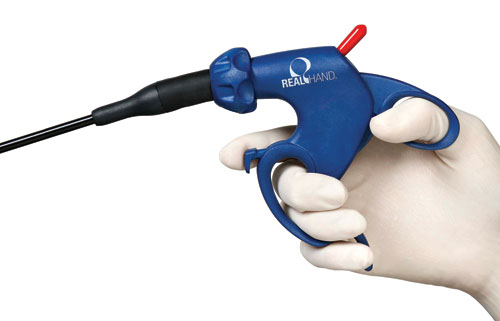
To improve upon the design of a competitive medical device, research was done to show how surgeons gripped the device, explore variations in surgeon hand sizes holding the device, examine the force surgeons routinely applied to the device, and how much space the devices occupied in the operating rooms. These observations helped the design team optimize the handle for comfort and ease-of-use in the operating room.
During the initial design research phase, cross-functional teams in Phillips-Medisize observed surgeons performing eight different procedures using competitors’ products at the Mayo Clinic. Over the course of two days in the field, the engineers also spoke with the surgeons, gathering insights on how to improve the customer’s version of the medical device.
The research showed how surgeons gripped the medical devices, the variations in surgeon hand sizes holding the devices, the force surgeons routinely applied to the devices, and how much space the devices occupied in the operating rooms. These observations were instrumental in helping the design team optimize the handle for comfort and ease-of-use in the operating room – high priorities in terms of fostering patient safety. These insights helped the team create a variety of ergonomic design options.
Each option took into account function, size, weight, cost, and form, which were arranged in priority. The pre-determined priorities helped the engineers provide quantitative analyses the customer could see at a glance with each initial design option.
For the customer’s benefit, the engineers also conducted product analysis and concept exploration sessions with manufacturing, tooling, and design engineers at the same table. The sessions, called Phillips JumpStart™, involve product teardown analysis and collaboration to identify areas of potential product enhancements, optimization, and savings.
Once the customer selected their design option of choice, the engineers refined the design and created an SLA prototype. All the while, the design and manufacturing teams collaborated closely with the customer to ensure their medical device design was optimized for manufacture and assembly. Early collaboration, combined with all key stakeholders’ upfront attention to detail, helped ensure optimal program timeline and cost efficiencies, a parity-plus medical device, and overall program success.
Phillips-Medisize
www.phillipsplastics.com
Filed Under: Medical-device manufacture





Tell Us What You Think!