For more than 20 years, Medtronic’s PillCam capsule endoscopy has been used as a minimally invasive, patient-friendly option for the detection of gastrointestinal diseases. Most recently, the company announced that the U.S. Food and Drug Administration (FDA) granted 510(k) clearance for its PillCam Small Bowel 3 system for remote endoscopy procedures. Working with Amazon logistics to provide both timely and accurate results, patients can now use the PillCam in the comfort of their homes and do not have to have the diagnostic analysis performed in a hospital, doctor’s office or clinic.
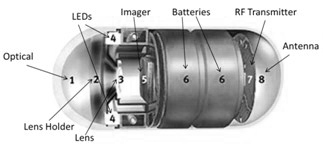
Measuring 11 mm x 26 mm and weighing less than four grams, the PillCam SB 3 contains an imaging device and light source and transmits images at a rate between two and six images per second. PillCam was initially cleared by the U.S. Food and Drug Administration in 2001. Other aspects of the earlier wireless endoscopy imaging capsule are shown below.
Image source: Understanding Smart Sensors, 3rd Edition.
Providing a telehealth option for direct visualization and monitoring of the small bowel, the greater need for remote diagnostic procedure of PillCam SB3 @HOME and its FDA approval are among the impacts of COVID 19.
“The pandemic necessitated more remote innovation, and our capabilities have exceeded expectations to provide better quality care to patients without the risk of COVID infection and without adding to the burden on the medical staff,” said Giovanni Di Napoli, president of the Gastrointestinal business, which is part of the Medical Surgical Portfolio at Medtronic.
Other improvements to the SB3 version include the use of adaptive frame rate technology that changes the capture image rate from 2 to 6 frames per second (fps) when the system determines the capsule is moving more quickly. In addition, the latest proprietary software algorithms enable smarter video compilation that is 40% more efficient than the previous SB2 system..
Filed Under: Sensor Tips