Patient safety advocates who have examined a hefty new proposed rule from the Centers for Medicare and Medicaid Services (CMS) say the agency is on course to eliminate data on infection rates from its quality reporting measures. Information on catheter-associated urinary tract infection (CAUTI), Clostridium difficile (C. Diff), postoperative sepsis, and other healthcare associated infections would no longer be part of the Inpatient Quality Reporting Program (IQR) and the government’s Hospital Compare website.
Leapfrog, the nonprofit organization that regularly releases hospital safety grades, quickly decried the proposed regulatory shift as a dangerous retreat on the principle of healthcare transparency. The watchdog group insists the reporting burden on hospitals is necessary to drive patient safety efforts.
“Among the reasons given in the proposed rule for removing infections from the IQR is the amount of time hospitals spend counting them, about 2 million hours,” Leapfrog notes in a press release. “Yet studies suggest that infections cost the economy $147 billion a year and untold suffering and grief.”
Jeanine Thomas, founder of the MRSA Survivors Network, tells USA Today that CMS is taking the wrong approach with their proposed rule..
“I am shocked that they want to reverse course on this,” she says. “In fact, they should do more.”
The American Hospital Association hasn’t weighed in on the proposed rule, but the organization has previously express concerns that the reporting requirements are overly burdensome.
The new rule still has an avenue for reporting infection rates and other patient safety metrics, but it is confined to a single Affordable Care Act program. According to those in opposition to the proposal, this would effectively make infection rate reporting voluntary. Hospitals struggling with infection prevention measures would likely decide paying a small fine for failing to submit numbers is preferable to the more widespread damage resulting from public exposure of facility problems.
“Even as the proposed rule would move these safety measures out of the IQR, it would also confine each measure to just one Obamacare payment program, meaning financial penalties for infections and errors would be reduced,” notes Leapfrog. “Infection and other safety measures should be included in all payment programs, because quality and cost-effectiveness are nullified when safety is absent.”
Kate Goodrich, the chief medical officer for CMS, tells USA Today that critics are misreading the proposal and insists the infection rate information won’t be removed from Hospital Compare under the new rule.
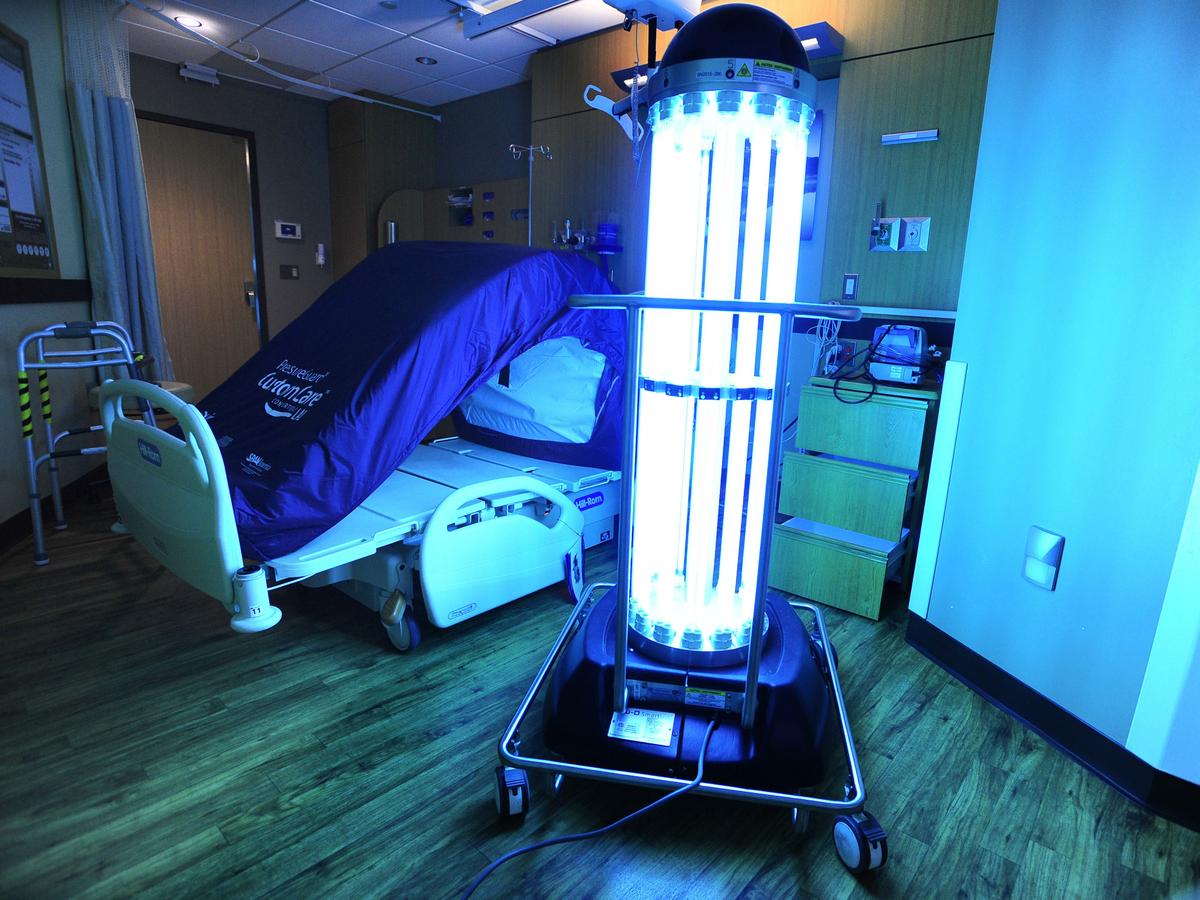
This 2017 photo shows the Tru-D SmartUVC in a hospital room in St. Charles Bend, Ore. (Ryan Brennecke/The Bulletin via AP)
The proposal arrives roughly concurrently with the CMS announcement that its hospital quality star ratings will not be updated in July, as was previously planned. Controversial from the beginning, the star ratings have often been bedeviled by delays.
“CMS has decided to postpone the July star ratings update to give time for additional analysis of the impact of changes to some of the measures on the star ratings and to address stakeholder concerns,” the federal agency explained. “When changes are made to the underlying measures it is vital to take the time needed to understand the impact of those changes and ensure we are giving consumers the most useful information.”
The proposed rule about hospital infection rate reporting is in a public comment period. If approved, it is expected to take effect in November.
Filed Under: Industry regulations




