
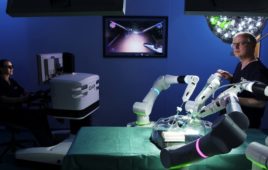
Corindus Vascular Robotics this week said it applied to the U.S. Food and Drug Administration for premarket clearance to use its CorPath GRX robotic surgical platform in neurovascular interventions.
The Waltham, Mass.-based company has already received FDA clearance for percutaneous coronary interventions (PCI), which it won in 2016, and for peripheral vascular interventions (PVI), which it won last year.
“The ability to treat neurovascular disease with CorPath GRX is the first step for physicians to gain critical experience with robotics,” stated Dr. Aquilla Turk, chief medical officer of Corindus Inc. “Applying the benefits of robotic precision to neurovascular intervention, while building a wealth of clinical knowledge and expertise, are key to preparing for a future of remote stroke treatment.”
Corindus claimed that if it receives the clearance, the CorPath GRX system would be the world’s first and only robotic platform indicated for all three indications — PCI, PVI, and neurovascular interventions or NVI.
“An expansion of CorPath GRX to treat neurovascular conditions would represent a major stepping stone towards our goal of revolutionizing stroke treatment,” said Mark Toland, Corindus president and CEO. “We believe that our current robotic platform will bring benefits to neurovascular procedures today.”
“We will leverage our recent achievements in telerobotics and strong focus on technology development to build a remote stroke solution to tackle the challenge of access to care,” he added. “This would extend the reach of highly-skilled specialists across the globe by granting remote access to patients suffering from life-altering diseases where access to care is limited and time to treatment is paramount.”
Last spring, Corindus raised $25 million in Series A funding to help commercialize the CorPath GRX system, and it received FDA approval for the Rotate on Retract proprietary software to control the CorPath GRX’s movement.
Last December, Corindus touted that its CorPath robotic surgical platform was used in a first in-human telerobotic intervention study in India.
Filed Under: The Robot Report, Robotics • robotic grippers • end effectors



Tell Us What You Think!